|
How to make a biological membrane in the lab
The Making of a Cell Membrane The making of a cell membrane is a remarkable wonder of the behavior
of biological molecules. It has been found that very large structures
in cells are made of many, sometimes up to thousands of units that
stick together without being tethered by chemical bonding. It is
the behavior of amphipathic molecules (part hydrophobic, part hydrophilic),
which tend to self-assemble in aqueous solution. In short, many
molecules spontaneously aggregate into regular structures. Phospholipids
aggregate into planar bilayers called membranes. The spontaneity
of the process has been exploited to reconstitute membranes in the
laboratory in the presence of membrane proteins. Two techniques,
still used today, have been developed in the 1960s and early 70s.
Both make use of a setup made of two Teflon half-chambers separated
by a very small hole. In the first case, the hole is covered by
a lipid solution (in decane) and the membrane will spontaneously
form while the organic solvent slowly dilutes into solution leaving
behind a phospholipid layer which at its thinnest part will be a
molecular bilayer. The formation can be monitored electronically
via capacitance measurements. The second technique makes use of
the apposition of two individual monolayers of phospholipids that
always form at the water surface. When covering the hole from both
sides by water covered with such a monolayer, the two monolayers
will get in contact through the hydrophobic fatty acid parts to
form a molecular bilayer with the same electronic properties as
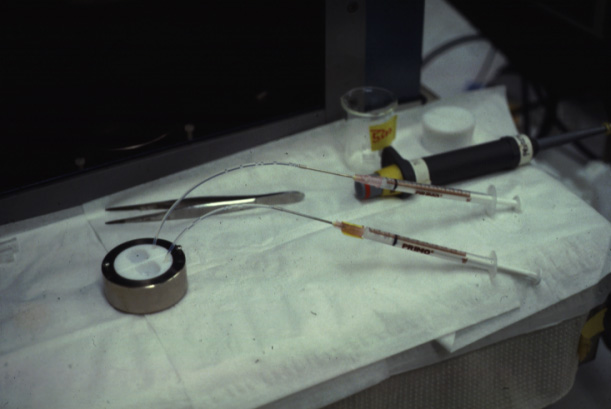
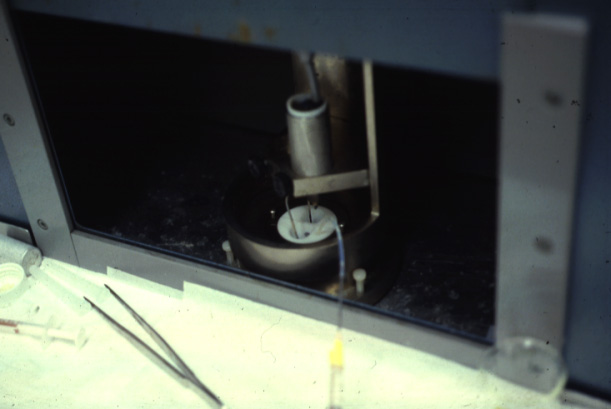
a bilayer formed by decane thinning. The following pictures show one of the earlier two chamber systems used by M. Montal at the University of California, San Diego.
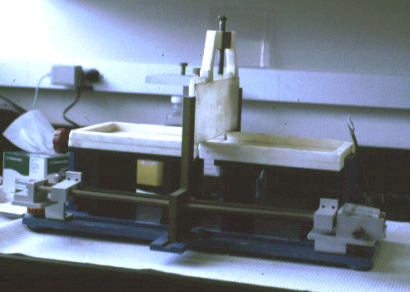
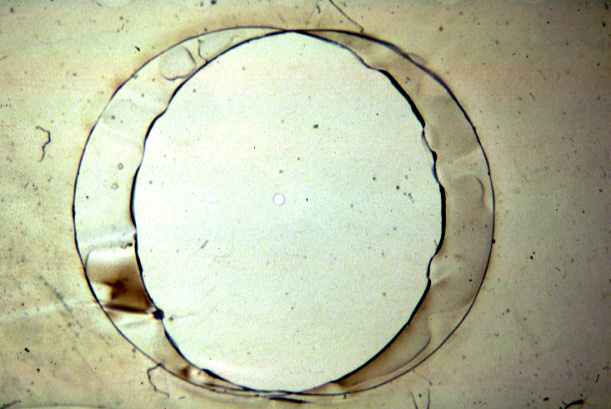
  (Photos Lukas Buehler) A refined version of the technique makes use of micrometer holes which are burned or cut into an extremely thin Teflon foil. H. Schindler developed the technique to use completely solvent free membranes to provide optimal recreation of a biological membrane.
These Teflon septum can be fixed between two small half-chambers
and each side of the partition can be filled with water and carefully
spread lipid monolayer on the surface. Using tubing connected to
syringes can be used to rise the water levels on both sides. The forming molecular bilayer across the aperture in the Teflon septum can be used to study ion channel activity, i.e., single channel recordings like the one shown above.
Copyright © 2000-2003 Lukas K. Buehler |
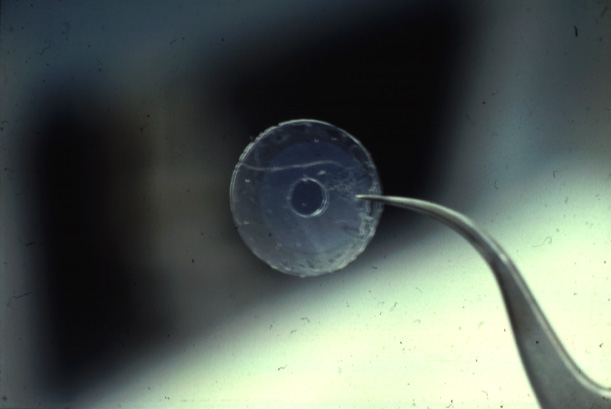
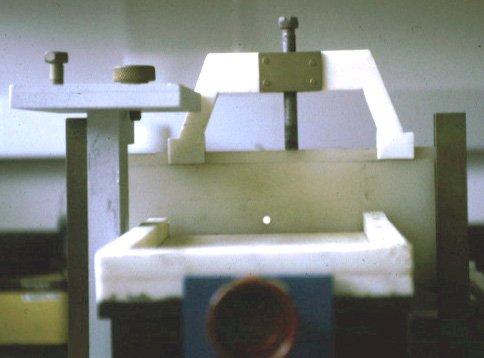
 (Teflon
septum (upper panel) with cut hole, diameter about 100micrometer
(lower panel);
(Teflon
septum (upper panel) with cut hole, diameter about 100micrometer
(lower panel);