Amino Acids
1. L-a -amino acids
Amino acids are the building blocks of proteins. All proteins are composed of 20 different amino acid types commonly obtained upon hydrolysis of proteins (>100 types have been isolated) and referred to as a-amino acids. Their general structure is shown below:
R
|
H2NóCa óCOOH
|
H
L - a - amino acid
The carbon atom can have 4 covalent bonds that are arranged in a tetrahedral manner. If the molecule is arranged in such a way that the H residue points upward from the carbon center and the three substituents COO-, R, and NH3+ spell CORN when read clockwise, the Fisher projection above of the chemical structure of an amino acid denotes an L-a -amino acid (horizontal lines Ė front of page; vertical lines Ė behind page).
Fig. Projection map to determine chirality of the alpha carbon center of a -amino acids.
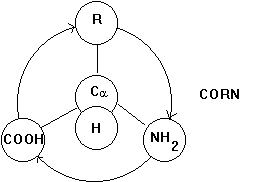
If all the substitute groups are chemically different, the Ca atom constitutes an optically active, or chiral, center, i.e., a solution of L-amino acid deflects the plane of polarized light by a certain angle. Changing the position of amino and carboxyl groups in the above structure changes the geometry of the molecule converting the L-amino acid into a D-amino acid. We have now two mirror symmetric molecules which are chemically equivalent, but structurally (stereochemically) different. There is no way that we can superimpose the two mirror symmetric molecules by simply rotating one over the other. The two molecules are referred to as enantiomeres. Biological systems often have high specificity for either one of a pair of enantiomeres, a phenomenon which we call stereo specific binding.
At physiological pH free amino acids are fully ionized, i.e., the carboxyl group looses its H+ and the amine group gains a H+. Although the molecules net charge is zero, it carries one positive and one negative charge giving it a zwitter ionic characteristic.:
Fig. Zwitter ionic L-amino acid at physiological pH 7.4
R
|
pK = 9.4 H3N+óCa
óCOO- pK = 2.2
|
H
The amino acids are ionized at physiological pH because the pK of the
carboxyl group is 2.2 and the pK(NH3+) = 9.4. The
pK represents the pH value at equilibrium, where 50% of the groups are
protonated and 50% are de-protonated. For both the carboxyl and the amine
group the physiological pH is far away from equilibrium.
2. The 20 naturally occurring L-amino acids
Amino acids differ in the side chains or amino acid residues, denoted
as 'R' in the above structures, determining the dielectric and electrochemical
properties of proteins. The amino acids commonly found in proteins have
one out of 20 different chemical groups that can be put into three major
categories with respect to their water solubility:
non-polar residuespolar, neutral residues
polar, charged residues
This classification reflects the polarity of the side chain and determines
the propensity of the side chain of being hydrated.
Various organisms on earth collectively synthesize an enormous number
of different proteins whose great range of physicochemical characteristics
stem largely from the varied properties of those 20 amino acids.
Table Classification and letter codes of naturally occurring L-amino
acids
| Quality | Amino acid | Mol. weight (Dalton) |
|
|
Hydro- * phobicity |
pKa |
| non-polar | Alanine | 89.1 |
|
|
+1.8 | |
| Valine | 117.15 |
|
|
+4.2 | ||
| Leucine | 131.18 |
|
|
+3.8 | ||
| Isoleucine | 131.18 |
|
|
+4.5 | ||
| Methionine | 149.21 |
|
|
+1.9 | ||
| Phenylalanine | 165.19 |
|
|
+2.8 | ||
| Glycine | 75.07 |
|
|
-0.4 | ||
| Tryptophane | 204.23 |
|
|
-0.9 | ||
| Proline | 151.13 |
|
|
-1.6 | ||
| polar (neutral) | Serine | 105.10 |
|
|
-0.8 | |
| Threonine | 119.12 |
|
|
-0.7 | ||
| Cysteine | 121.16 |
|
|
+2.5 | ||
| Asparagine | 132.12 |
|
|
-3.5 | ||
| Glutamine | 128.10 |
|
|
-3.5 | ||
| Tyrosine | 181.19 |
|
|
-1.3 | ||
| polar (charged) | Lysine | 146.19 |
|
|
-3.9 | 10.54 |
| Arginine | 174.2 |
|
|
-4.5 | 12.48 | |
| Histidine | 155.16 |
|
|
-3.2 | 6.04 | |
| Aspartate | 133.11 |
|
|
-3.5 | 3.90 | |
| Glutamate | 147.13 |
|
|
-3.5 | 4.07 |
* hydrophobicity: positive values indicate hydrophopic amino acids, negative
values hydrophilic amino acids (after Kyte&Doolittle, 1982); some
amino acids are difficult to categ0rize because they are weakly hydrophobic
or are amphipathic, meaning having strong local polar or non-polar bond
structures; cysteine is not very water soluble, but can form weak H-bonds
and has a reacitve outer electron shell
Fig. Chemical structures of the 20 L-amino acids used for protein
biosynthesis
Glycine (Gly)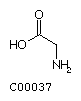 Alanine (Ala)
Alanine (Ala)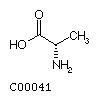 Valine
(Val)
Valine
(Val)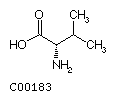
Leucine (Leu)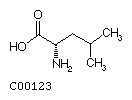 Isoleucine (Ile)
Isoleucine (Ile)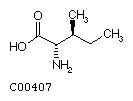
Aspartate (Asp)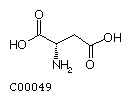 Asparagine (Asn)
Asparagine (Asn)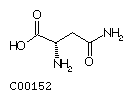
Glutamate (Glu)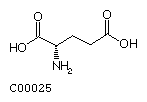 Glutamine (Gln)
Glutamine (Gln)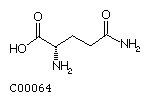
Serine (Ser)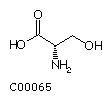 Threonine (Thr)
Threonine (Thr)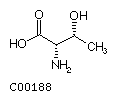
Methionine (Met)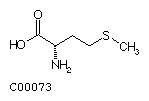 Cysteine (Cys)
Cysteine (Cys)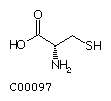
Lysine (Lys)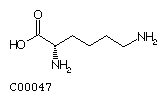 Arginine (Arg)
Arginine (Arg)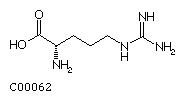
Histidine (His)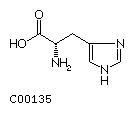 Proline (Pro)
Proline (Pro)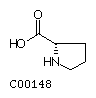
Phenylalanine (Phe)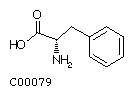 Tyrosine (Tyr)
Tyrosine (Tyr)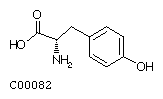
Tryptophan (Trp)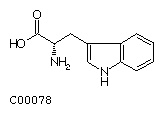
3. Amino acid derivatives
Many more amino acids can be found in proteins, two of which occur in the extracellular protein collagen. These amino acids are chemically modified after the protein has been synthesized. This is called post-translational modification. The two post-translationally modified amino acids in collagen are Hydroxyproline and Hydroxylysine.
Fig. Chemical structure of 4-hydroxyproline and 5-hydroxylysine
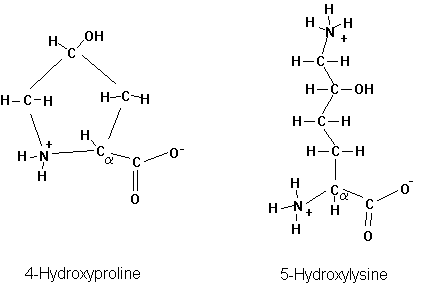
Ribosomal proteins and histones, responsible in protein synthesis and chromosome structure, respectively, contain many methylated, and acetylated amino acids. The most common post translational modification is the phosphorylation of proteins (on serines, threonines, and tyrosines). Phosphorylation is a major signal transduction mechanism as well as energy delivery system for enzyme activity.
Some microorganisms make use of D-amino acids. The presence of these
D-amino acids is also the result of posttranslational modification, a
process called stereoisomerization, where the alpha hydrogen and side
chain change their respective position in the tetrahedral bond geometry
of the a carbon center.